Unraveling Molecular Mechanisms for Efficient Intracellular Transport – Potential Applications in Novel Drug Delivery System Development –
Points of this study
- Molecular “Zip-codes” information necessary for the correct delivery of trafficking vesicles has not been well understood.
- It has been discovered that Rab GTPase proteins and lipids called phosphoinositides, in coordination with SNARE proteins, act as “Zip-codes”, enabling the precise transport of trafficking vesicles.
- The findings of this study could lead to the development of a new pharmaceutical delivery system that delivers drugs to specific locations within cells.
Summary
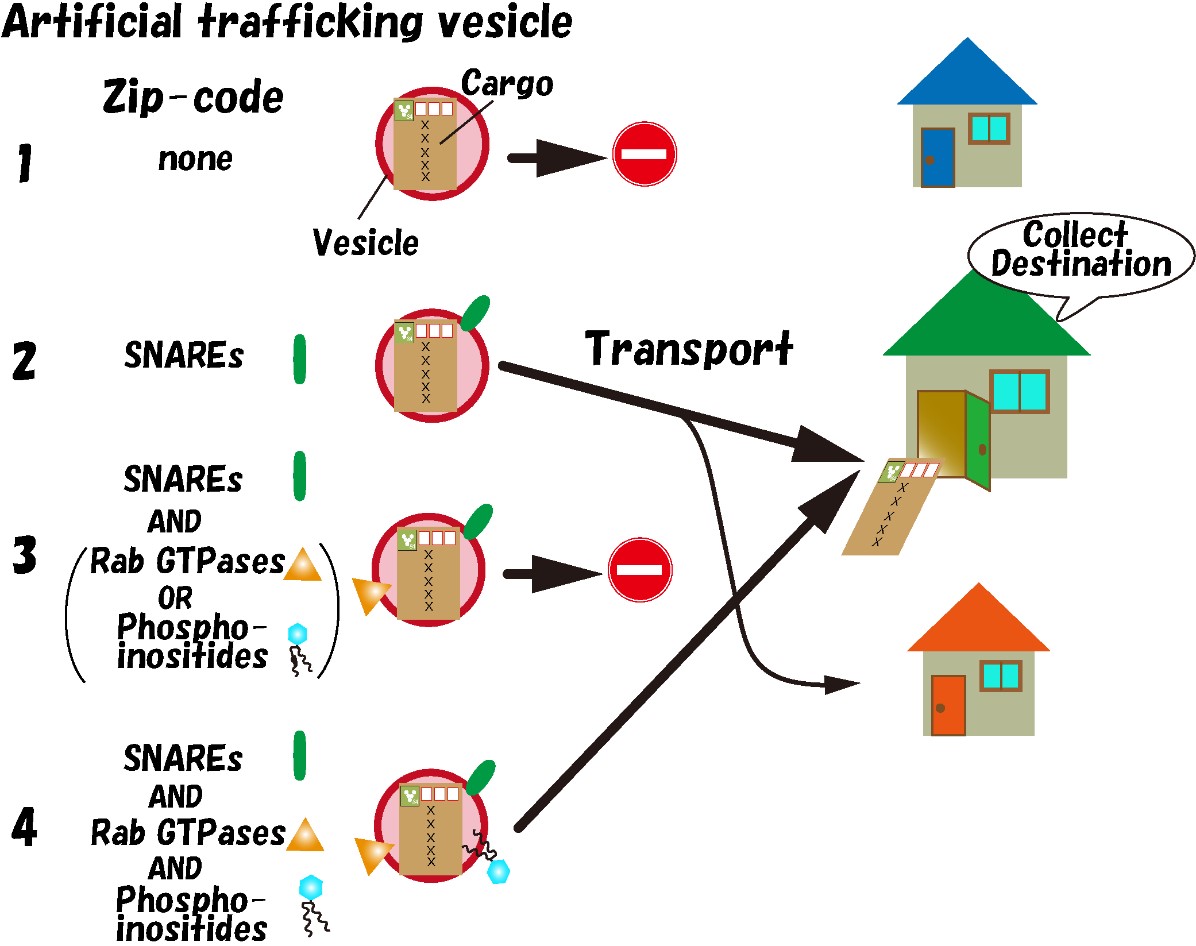
Within cells, there exist numerous organelles, such as the endoplasmic reticulum, the Golgi apparatus, and lysosomes, which are composed of membranous structures. These organelles exchange materials using smaller vesicles called trafficking vesicles. Accurate delivery of trafficking vesicles to their target organelles is essential for cellular survival. While it has been thought that “Zip-code” information is imprinted on trafficking vesicles, the molecular nature of this information has not been elucidated.
An international research group led by Associate Professor Seiichi Koike of the Faculty of Engineering at the University of Toyama and Professor Reinhard Jahn of the Department of Neurobiology at the Max Planck Institute for Multidisciplinary Sciences in Germany has developed a new method to inject artificial trafficking vesicles into cells and observe their behavior (Koike and Jahn 2017 PNAS). Using this method, they found that SNARE proteins act as destination information and that the destination of vesicles changes depending on the subtype of SNARE protein on the vesicles (Koike and Jahn 2019 Nature Communications). However, it was suggested that factors other than SNARE proteins may be involved in determining the destination, as artificial trafficking vesicles containing only SNARE proteins showed lower specificity in vesicle targeting compared to endogenous trafficking vesicles.
In this study, the group focused on molecules other than SNARE proteins, and they found that Rab GTPases and phosphoinositides play a significant role in determining the destination of vesicles. In liposomes containing SNARE proteins and either active Rab GTPase proteins or phosphoinositides, transport based on the “Zip-codes” information by SNARE proteins was suppressed, and vesicles were no longer transported anywhere. However, when all three types of molecules (active Rab GTPase proteins, phosphoinositides, and SNARE proteins) were bound to the liposomes, the specificity of the destination increased compared to when SNARE proteins alone were present. This effect was not observed when inactive form of Rab GTPases was used, indicating a dependence on the activity of Rab GTPase. From these results, it was revealed that Rab GTPases and phosphoinositides function as the initiation switch for transport, and if only one of them is present, the “Zip-codes” information by SNARE proteins is suppressed, and vesicles can be transported to their destination only when both are present. Furthermore, active Rab GTPases and phosphoinositides play a role in enhancing the accuracy of vesicle destination.
Recently, the development of drug delivery systems aimed at delivering drugs to specific cells is progressing. The results of this study could lead to the development of a novel system for delivering drugs specifically to organelles. This “organelle” specific drug delivery system would be potentially enhancing the effectiveness of drugs.
Published Details
Title
Rab GTPases and phosphoinositides fine-tune SNAREs dependent targeting specificity of intracellular vesicle traffic
Authors
Seiichi Koike and Reinhard Jahn
Published in
Nature Communications

